Methodological expertise :
Research typology, calculation of required sample size, protocol writing.
Project management :
Coordination, trial oversight, recruitment management and optimization, trial monitoring.
Data Management & Statistics :
Data Management Plan, Statistical Analysis Plan, data processing, statistical analyses, statistical report.
Design and writing of documents :
Protocol, investigator brochure, information sheet, data collection form, and case report form.

Our expertise
Site Qualification for Research
University hospitals, private clinics, private practices, pharmacies.
On-site Feasibility
Provision of the most suitable sites for your project (therapeutic area, scientific reputation, recruitment potential, human and material resources).
Site Management
Site selection and qualification, site initiation visits, on-site and remote monitoring visits, monitoring reports, close-out visits, investigator support.
Electronic Data Collection Tools Configuration
eCRF, ePRO, eConsent.
Regulatory Authorities
ANSM (French National Agency for Medicines), CPP (Ethics Committees), CNIL (French Data Protection Authority), Health Data Hub.
Financial Agreements
Negotiation of hospital costs/overheads and drafting of financial agreements.
Regional Health Agencies and Professional Orders
ARS, CNOM (via the IDAHE2 platform).
Financial Agreements
Negotiation of hospital costs/overheads and drafting of financial agreements.
International Registries
ClinicalTrials.gov, CTIS (Clinical Trials Information System).
Médi-Link since 2016
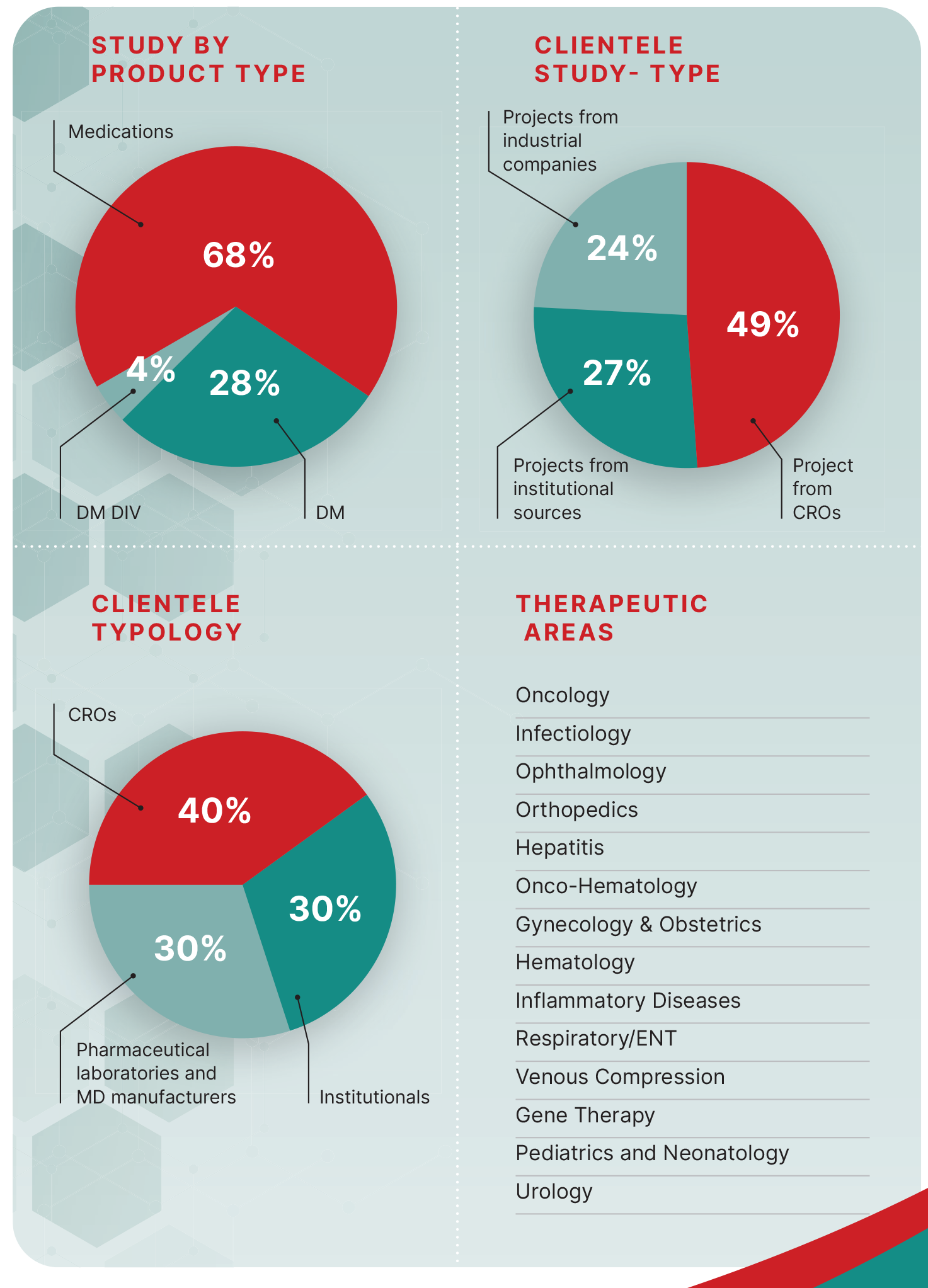
STUDIES BY PRODUCT TYPE
68% Drugs
28% Medical Devices (MD)
4% In Vitro Diagnostics (IVD)
NUMBER OF STUDIES BY CLIENT TYPE
49% from CROs
27% from institutional clients
24% from industry sponsors
CLIENT TYPOLOGY
40% Pharmaceutical companies and medical device manufacturers
30% Institutional clients
30% CROs
Our Ambition for 2030
To become a key leader in supporting hospitals, pharmaceutical laboratories, and medical device manufacturers.
Our Services and Values
With us, you benefit from an optimized, unified approach that is perfectly tailored to your needs—without complicating communication, multiplying contacts, or increasing your budget
Reliability
Expertise, Quality of work, Guidance, Client satisfaction — including physicians, but above all, patients.
Responsiveness
Clarity of missions, region-based employees, availability, local networks, quick feedback, clearly defined roles.
Commitment
Team spirit, involvement, respect for key performance indicators, mutual support, kindness, quality of work life, work-life balance.
OUR MISSION
Restoring trust among all stakeholders, ensuring the quality of your clinical studies, and securing the success of your initiatives — even under critical timelines.












